Most traditional pharmaceuticals are relatively low molecular weight substances and generally escape the attention of the immune system. Proteins, on the other hand, are macromolecules and display molecular properties that can potentially trigger a vigorous immune response. During its formation our immune system develops tolerance to self antigens. Such immunological tolerance is generally maintained throughout our lifetime by various regulatory mechanisms that either :-
Most traditional pharmaceuticals are relatively low molecular weight substances and generally escape the attention of the immune system. Proteins, on the other hand, are macromolecules and display molecular properties that can potentially trigger a vigorous immune response. During its formation our immune system develops tolerance to self antigens. Such immunological tolerance is generally maintained throughout our lifetime by various regulatory mechanisms that either :-i) Prevent B- and T-lymphocytes from becoming responsive
to self-antigens.
OR
ii) That inactivate such immune effector cells once they
encounter self-antigens.
So why do therapeutic proteins of human amino acid sequences have the potential to trigger an immune response?
Potential reasons can include:
1) Differences in post-translational modification.
Human therapeutic proteins produced in several recombinant systems (e.g. yeast, plant and insect based systems) can display altered post translational modification detail, particularly in the context of glycosylation. Some sugar residues/motifs characteristic of these systems can be highly immunogenic in humans.
2) Structural alteration of the protein during processing
or storage.
Suboptimal product processing or formulation can result in partial degradation, denaturation, aggregation or precipitation of the therapeutic protein. Epitopes normally shielded from immune surveillance may be exposed as a result, triggering an immune response.
3) Dosage levels and duration of treatment.
High dosage levels (well above normal physiological ranges), in particular if a product is administered on an ongoing and regular basis, may potentially contribute to breaking self-tolerance, particularly if combined with any of the circumstances outlined in the surrounding bulleted points.
4) Genetic or immunological factors.
Some individuals may display underlining or induced immunological abnormalities, rendering them more susceptible to breakdown of self tolerance. For example, some blood factor and hormone preparations isolated by direct extraction from human serum or tissue stimulated an immunological response in a proportion of human patients receiving them. This may be triggered by some immune deficiency in the patients themselves, although the presence of product impurities or structural altered product forms may also be contributing factors.
Even if a biopharmaceutical triggers an immune response, it does not automatically follow that the response will be clinically significant or undesirable. In some instances, anti-product antibodies have no effect upon safety or efficacy. In other instances, antibody binding may alter the product’s pharmacokinetic properties or directly neutralize the biopharmaceutical’s biological activity. Even more seriously, antibodies raised against the product could potentially cross-react with the endogenous form of the protein, neutralizing it. Eprex provides an example of this latter phenomenon. Antibodies formed against the product cross-reacted with endogenous EPO, causing shutdown of (EPO-stimulated) red blood cell production, triggering antibody-mediated pure red cell aplasia.
A number of approaches may be adopted in an attempt to reduce or eliminate protein immunogenicity. Protein engineering, for example, has been employed to humanize monoclonal antibodies. An alternative approach entails the covalent attachment of polyethylene glycol (PEG) to the protein backbone. This can potentially shield immunogenic epitopes upon the protein from the immune system.
Some individuals may display underlining or induced immunological abnormalities, rendering them more susceptible to breakdown of self tolerance. For example, some blood factor and hormone preparations isolated by direct extraction from human serum or tissue stimulated an immunological response in a proportion of human patients receiving them. This may be triggered by some immune deficiency in the patients themselves, although the presence of product impurities or structural altered product forms may also be contributing factors.
Even if a biopharmaceutical triggers an immune response, it does not automatically follow that the response will be clinically significant or undesirable. In some instances, anti-product antibodies have no effect upon safety or efficacy. In other instances, antibody binding may alter the product’s pharmacokinetic properties or directly neutralize the biopharmaceutical’s biological activity. Even more seriously, antibodies raised against the product could potentially cross-react with the endogenous form of the protein, neutralizing it. Eprex provides an example of this latter phenomenon. Antibodies formed against the product cross-reacted with endogenous EPO, causing shutdown of (EPO-stimulated) red blood cell production, triggering antibody-mediated pure red cell aplasia.
A number of approaches may be adopted in an attempt to reduce or eliminate protein immunogenicity. Protein engineering, for example, has been employed to humanize monoclonal antibodies. An alternative approach entails the covalent attachment of polyethylene glycol (PEG) to the protein backbone. This can potentially shield immunogenic epitopes upon the protein from the immune system.
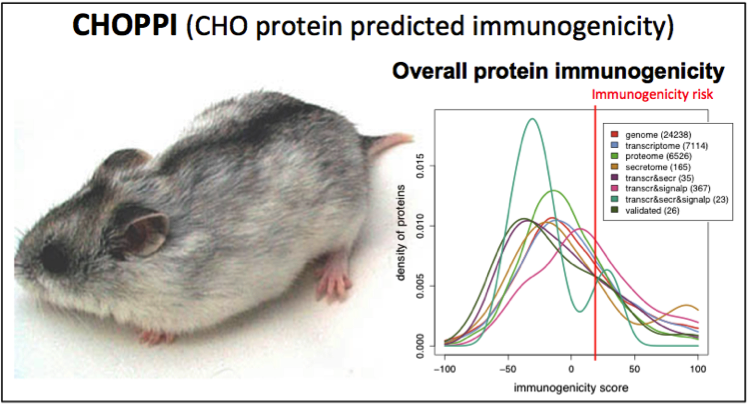




.gif)