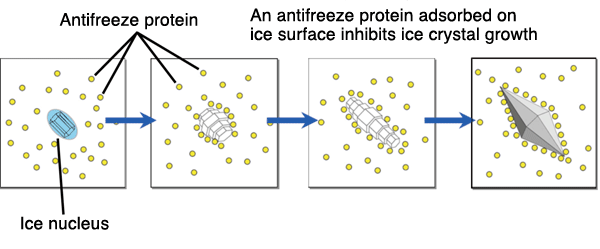
 Antifreeze proteins (AFPs) or ice structuring proteins (ISPs) refer to a class of polypeptides produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind to small ice crystals to inhibit growth and recrystallization of ice that would otherwise be fatal. There is also increasing evidence that AFPs interact with mammalian cell membranes to protect them from cold damage. This work suggests the involvement of AFPs in cold acclimatization.
Antifreeze proteins (AFPs) or ice structuring proteins (ISPs) refer to a class of polypeptides produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind to small ice crystals to inhibit growth and recrystallization of ice that would otherwise be fatal. There is also increasing evidence that AFPs interact with mammalian cell membranes to protect them from cold damage. This work suggests the involvement of AFPs in cold acclimatization. HISTORY
In the 1950s, Canadian scientist scholander set out to explain how Arctic Fish can survive in water colder than the freezing point of their blood. His experiments led him to believe that there was some kind of "antifreeze" material in the blood of Arctic Fish. Then in the late 1960s, animal biologist Arthur De Vries was able to isolate the antifreeze protein through his ivestigation of Antarctic Fish.
NON-COLLIGATIVE PROPERTIES
Unlike the widely used automotive antifreeze, ethylene glycol, AFPs do not lower freezing point in proportion to concentration. Rather, they work in a non-colligative manner. This allows them to act as antifreeze at concentrations 1/300th to 1/500th of those of other dissolved solutes. This minimizes their effect on osmotic pressure. The unusual capabilities of AFPs are attributed to their binding ability at specific ice crystal surfaces.
THERMAL HYSTERESIS
AFPs create a difference between the melting point and freezing point known as thermal hysteresis. The addition of AFPs at the interface between solid ice and liquid water
inhibits the thermodynamically favoured growth of the ice crystal. Ice growth is kinetically inhibited by the AFPs covering the water-accessible surfaces of ice. Thermal hysteresis is easily measured in the lab with a nanolitre ohmmeter. Different organisms have different values of thermal hysteresis. The maximum level of thermal hysteresis shown by fish AFP is approximately -1.5°C (29.3°F). However, insect antifreeze proteins are 10–30 times more active than any known fish protein.
CLASSIFICATION OF AFP CONTAINING SPECIES
Species containing AFPs may be classified as:
Freeze avoidant: These species are able to prevent their body fluids from freezing altogether. Generally, the AFP function may be overcome at extremely cold temperatures, leading to rapid ice growth and death.
Freeze tolerant: These species are able to survive body fluid freezing. Some freeze tolerant species are thought to use AFPs as cryoprotectants to prevent the damages of freezing; It inhibits recrystallization and stabilizes cell membranes to prevent damage by ice.
MECHANISM OF ACTION
AFPs are thought to inhibit growth by an adsorption–inhibition mechanism. They adsorb to non-basal planes of ice, inhibiting thermodynamically favoured ice growth. The presence of a flat, rigid surface in some AFPs seems to facilitate its interaction with ice via Van der Waals force surface complementarily.
- Binding to ice
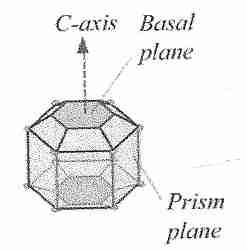 Normally, ice crystals grown in solution only exhibit the basal (0001) and prism faces (1010), and appears as round and flat discs. However, it appears the presence of AFPs exposes other faces. It now appears the ice surface 2021 is the preferred binding surface, at least for AFP type I. Through studies on type I AFP, ice and AFP were initially thought to interact through hydrogen bonding (Raymond and DeVries, 1977). However, when parts of the protein thought to facilitate this hydrogen bonding were mutated, the hypothesized decrease in antifreeze activity was not observed. Recent data suggest hydrophobic interactions could be the main contributor. It is difficult to discern the exact mechanism of binding because of the complex water-ice interface. Currently, attempts to uncover the precise mechanism are being made through use of molecular modelling Programmes.
Normally, ice crystals grown in solution only exhibit the basal (0001) and prism faces (1010), and appears as round and flat discs. However, it appears the presence of AFPs exposes other faces. It now appears the ice surface 2021 is the preferred binding surface, at least for AFP type I. Through studies on type I AFP, ice and AFP were initially thought to interact through hydrogen bonding (Raymond and DeVries, 1977). However, when parts of the protein thought to facilitate this hydrogen bonding were mutated, the hypothesized decrease in antifreeze activity was not observed. Recent data suggest hydrophobic interactions could be the main contributor. It is difficult to discern the exact mechanism of binding because of the complex water-ice interface. Currently, attempts to uncover the precise mechanism are being made through use of molecular modelling Programmes.- Binding Mechanism and Antifreeze Function
According to the structure and function study on the antifreeze protein from the fish winter flounder, the antifreeze mechanism of the type-I AFP molecule was shown to be due to the binding to an ice nucleation structure in a zipper-like fashion through hydrogen bonding of the hydroxyl groups of its four The residues to the oxygen along the direction in ice lattice, subsequently stopping or retarding the growth of ice pyramidal planes so as to depress the freeze point.
The above mechanism can be used to elucidate the structure-function relationship of other antifreeze proteins with the following two common features:
- Recurrence of a The residue (or any other polar amino acid residue whose side-chain can form a hydrogen bond with water) in an 11-amino-acid period along the sequence concerned.
- A high percentage of an Ala residue component therein.
COMMERCIAL APPLICATIONS
- Increasing freeze tolerance of crop plants and extending the harvest season in cooler climates.
- Improving farm fish production in cooler climates.
- Lengthening shelf life of frozen foods.
- Improving Cryosurgery.
- Enhancing preservation of tissues for transplant or transfusion in medicine.
- Therapy for Hypothermia.
RECENT NEWS
One recent, successful business endeavor has been the introduction of AFPs into ice cream and yogurt products. This ingredient, labelled ice-structuring protein, has been approved by the Food and Drug Administration. The proteins are isolated from fish and replicated, on a larger scale, in yeast.
There is concern from organizations opposed to Genetically Modified Organisms (GMOs), arguing modified antifreeze proteins may cause inflammation. Intake of AFPs in diet is likely substantial in most northerly and temperate regions already. Given the known historic consumption of AFPs, it is safe to conclude their functional properties do not impart any toxicological or allergenic effects in humans. Currently, Unilever incorporates AFPs into some of its American products, including some popsicles and a new line of Breyers Light Double Churned ice cream bars. In ice cream, AFPs allow the production of very creamy, dense, reduced fat ice cream with fewer additives. They control ice crystal growth brought on by thawing on the loading dock or kitchen table which drastically reduces texture quality.
Stability of superheated water ice crystals in an AFP solution, while the proteins can inhibit freezing, they can also inhibit melting.


No comments:
Post a Comment