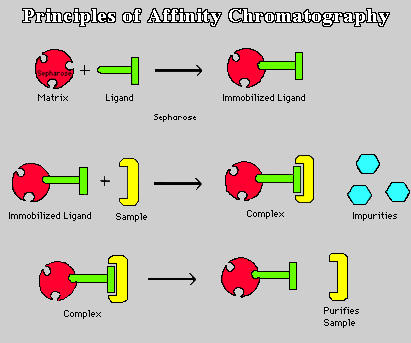
The Chromatography which allows the separation of biochemical mixtures based on a highly specific interaction such as that between antigen and antibody, enzyme and substrate or receptor and ligand is called AFFINITY CHROMATOGRAPHY.
Affinity chromatography is often described as the most powerful highly selective method of protein purification available. This technique relies on the ability of most proteins to bind specifically and reversibly to other compounds, often termed ligands. A wide variety of ligands may be covalently attached to an inert support matrix, and subsequently packed into a chromatographic column. In such a system, only the protein molecules that selectively bind to the immobilized ligand will be retained on the column. Washing the column with a suitable buffer will flush out all unbound molecules. An appropriate change in buffer composition, such as inclusion of a competing ligand, will result in desorption of the retained proteins.
Elution of bound protein from an affinity column is achieved by altering the composition of the elution buffer, such that the affinity of the protein for the immobilized ligand is greatly reduced. A variety of non-covalent interactions contribute to protein ligand interaction. In many cases, changes in buffer pH, ionic strength, inclusion of a detergent or agents such as ethylene glycol (which reduce solution polarity) may suffice to elute the protein. In other cases, inclusion of a competing ligand promotes desorption. Competing ligands often employed include free substrates, substrate analogues or cofactors. Use of a competing ligand generally results in more selective protein desorption than does a generalized approach such as alteration of buffer pH or ionic strength. In some cases, a combination of such elution conditions may be required. Identification of optimal desorption conditions often requires considerable empirical study.
Affinity chromatography offers many advantages over conventional chromatographic techniques. The specificity and selectivity of biospecific affinity chromatography cannot be matched by other chromatographic procedures. Increases in purity of over 1000-fold, with almost 100 percent yields, are often reported, at least on a laboratory scale. Incorporation of an affinity step could thus drastically reduce the number of subsequent steps required to achieve protein purification. This, in turn, could result in dramatic time and cost savings, which would be particularly significant in an industrial setting.
Despite such promise, biospecific affinity chromatography does display some practical limitations :
Affinity chromatography is often described as the most powerful highly selective method of protein purification available. This technique relies on the ability of most proteins to bind specifically and reversibly to other compounds, often termed ligands. A wide variety of ligands may be covalently attached to an inert support matrix, and subsequently packed into a chromatographic column. In such a system, only the protein molecules that selectively bind to the immobilized ligand will be retained on the column. Washing the column with a suitable buffer will flush out all unbound molecules. An appropriate change in buffer composition, such as inclusion of a competing ligand, will result in desorption of the retained proteins.
Elution of bound protein from an affinity column is achieved by altering the composition of the elution buffer, such that the affinity of the protein for the immobilized ligand is greatly reduced. A variety of non-covalent interactions contribute to protein ligand interaction. In many cases, changes in buffer pH, ionic strength, inclusion of a detergent or agents such as ethylene glycol (which reduce solution polarity) may suffice to elute the protein. In other cases, inclusion of a competing ligand promotes desorption. Competing ligands often employed include free substrates, substrate analogues or cofactors. Use of a competing ligand generally results in more selective protein desorption than does a generalized approach such as alteration of buffer pH or ionic strength. In some cases, a combination of such elution conditions may be required. Identification of optimal desorption conditions often requires considerable empirical study.
Affinity chromatography offers many advantages over conventional chromatographic techniques. The specificity and selectivity of biospecific affinity chromatography cannot be matched by other chromatographic procedures. Increases in purity of over 1000-fold, with almost 100 percent yields, are often reported, at least on a laboratory scale. Incorporation of an affinity step could thus drastically reduce the number of subsequent steps required to achieve protein purification. This, in turn, could result in dramatic time and cost savings, which would be particularly significant in an industrial setting.
Despite such promise, biospecific affinity chromatography does display some practical limitations :
- Many biospecific ligands are extremely expensive and often exhibit poor stability.
- Many of the ligand coupling techniques are chemically complex, hazardous, time consuming and costly.
- Any leaching of coupled ligands from the matrix also gives cause for concern for two reasons, as (a) it effectively reduces the capacity of the system and (b) leaching of what are often noxious chemicals into the protein products is undesirable.
It is not normally prudent to employ biospecific affinity chromatography as an initial purification step, as various enzymatic activities present in the crude fractions may modify or degrade the expensive affinity gels. However, it should be utilized as early as possible in the purification procedure in order to accrue the full benefit afforded by its high specificity.

No comments:
Post a Comment